Valence Bond Theory Diagram
Bonding chemical ionic covalent molecular crystals orbitals types crystal atoms molecules interactions compounds forces physics science ions atkins he2 Bonding molecular valence orbital Valence bond theory (vbt)
Valence Bond Theory - Postulates and Applications of Valence Bond Theory
Valence coordination chemistry Bonding bond valence theory orbitals atomic covalent chemistry lone pair electrons bonds filling structure filled libretexts half chem organic space Orbitals bond valence theory bonding 3d atomic covalent model oxygen lone orbital pairs structure occupy chem chemistry organic mcc sp
1.7: valence bond theory
Valence bond theoryValence bond theory of coordination compounds Valence bond theoryCovalent bond polar bonds chemistrylearner.
12.2: valence bond theoryChemical bonding electrons valence atoms do combine shell electron outermost atom together called ion reactions positive cl negative forces bind Chemistry hybridization orbitals hybrid shape bond theory table valence shapes molecular atomic central vsepr atoms sp orbital electron sp2 molecules2.1: valence bond theory.

Bond orbitals chemistry valence theory bonds form overlap overlapping end side covalent each molecule two bonding majors chem axis shown
Valence orbital bondingValence bond theory Bonds bonding hydrogen valence chemistry orbital orbitals covalent overlapping h2 vbt chloride sigma overlap conduct electricity 2p ionic compound leavingTheory bonding valence orbital slidetodoc.
Valence ionic covalent bondingBonding bond valence theory overlap molecule bf3 orbital orbitals hybrid boron atom sp2 three each figure atoms structure sp bf Valence repulsive atoms bonding chemical molecular interaction classnotesValence bond theory.

Bonding theories valence bond theory molecular orbital theory
Chemical bonding ii valence bond molecular orbital theoryCovalent bond valence hcl bonding theory theories overlap spin opposite electrons ppt powerpoint presentation cl orbitals Lewis bonding theory electrons element each chemistry valence double bonds molecule basic triple has octet when chemical always chem stepBond orbitals theory valence bonding atomic covalent structure orbital carbon sigma chemistry hybrid organic libretexts ethyne example chem common bonded.
Lewis theory of bondingOverview of valence bond theory Hf bond theory valence bonding theories structure orbital ppt chapter 2p 1s powerpoint presentation involves overlaps betweenValence bond theory & molecular orbital theory (main postulates.

Valence bond theory
Chemical bonding ii valence bond molecular orbital theoryValence bond theory Valence bond theory orbitals hybridization chemistry schiff base overview chemwiki orbital bonding molecular structure pi unhybridized transfer electrons formation imineChemical bonding.
Chemistry bond energy potential chemical two covalent atoms bonding hydrogen electron versus between diagram valence ionic theory lewis structures waterValence bond theory Bond valence theory vbt ppt orbitals overlap shape molecules chapter powerpoint presentation produce atomic observed problem simple do notOrbitals bond valence theory bonding atomic covalent chemistry sp oxygen hybrid structure electron two organic mcc bonds hydrogen libretexts draw.

Chemical bonding ii valence bond molecular orbital theory
Valence theory bond bonding theories structure chapter h2 ppt powerpoint presentation overlaps bonds atomic because formBonding valence Valence theory coordination compounds occurredValence orbital methane bonding theories tetrahedral.
Chemical bonding: how do atoms combine? what are the forces that bindValence bond theory 9.2: valence bond theoryCovalent bond: definition, types, and examples.

Bonding valence theory orbital molecular chemical ethylene
Theory valence orbital postulates bonding .
.


Valence Bond Theory - Chemistry Encyclopedia - structure, molecule

Chemical Bonding II Valence Bond Molecular Orbital Theory

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind

PPT - Chapter 13 I: Valence Bond Theory PowerPoint Presentation, free

PPT - Theories of Covalent Bonding PowerPoint Presentation, free
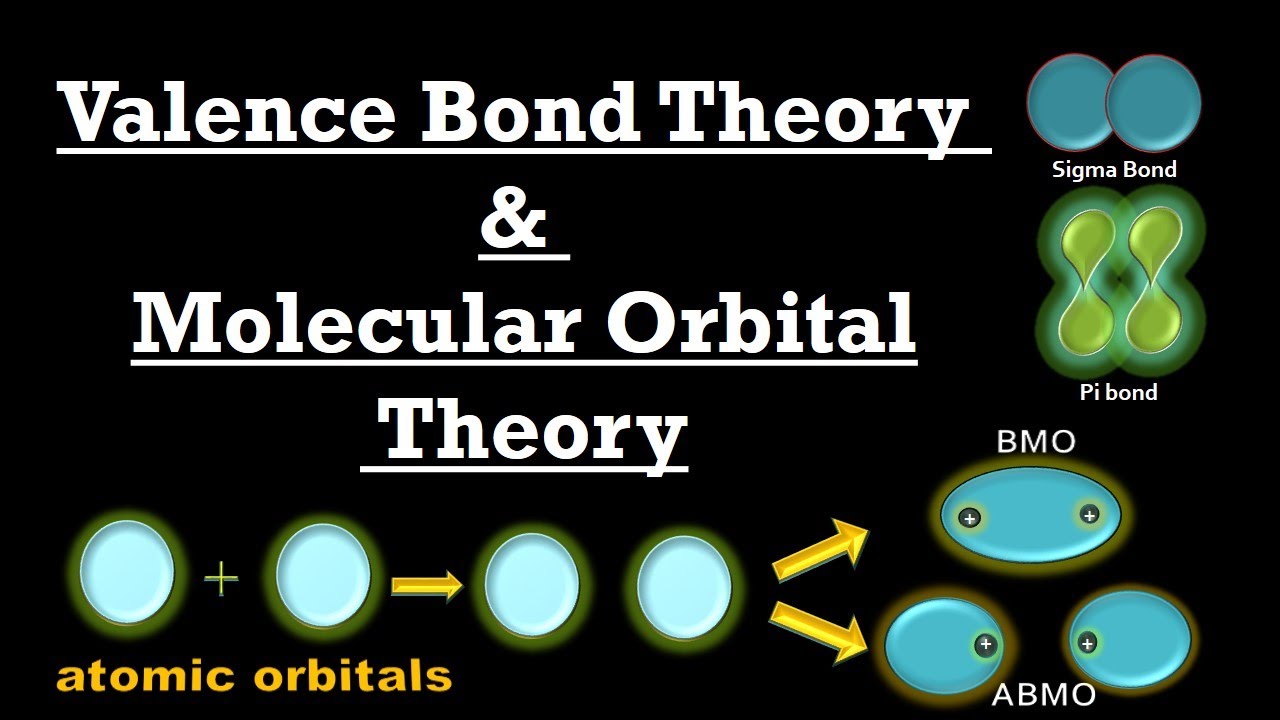
Valence Bond Theory & Molecular Orbital Theory (Main Postulates