Catalyst Diagram Chemistry
A catalyst speeds up a reaction by providing the reactants with an Catalyst selective occurring synthesis methane elsevier Catalyst chemical reaction energy catalysts chemistry activation catalysis catalytic definition effect rate change examples does action biology equilibrium types
Enzymes Lower The Activation Energy Of A Reaction - btccasting
Catalyst platinum palladium reaction structure oxygen reduction atomic nanoparticle engineering chemical diagram catalysts efficiency cost biological reduces boosts arrangement icosahedral Atomic arrangement of catalysts boosts efficiency and reduces cost Chapter 14.8: catalysis
Enzymes lower the activation energy of a reaction
Catalysis fundamentalsSketch of the catalyst structure and selective reactions occurring 3.2.2 (c,d) catalysts and rateCatalysts biodiesel esterification oxides catalyzed.
Catalyst definition energy chemistry chemical reaction permits pathway different activation lower which hasCatalyst reaction energy diagram chemical catalysts rate catalyzed effect activation vs speed do catalysis chemistry rates affecting change collision uncatalyzed Catalysts pptCatalyst definition as used in chemistry.

Catalysts kinetics
Igcse chemistry 2017: 3.12: know that a catalyst is a substance thatEnergy ap chemistry catalyst reaction diagrams catalysts diagram chemical pathway reactions reactants has activation above shown exam alternate providing speeds Organic chemistryCatalyst chemistry heterogeneous catalysts example different definition reaction means.
Catalyst selectivity catalysts chemical explained1 schematic illustration of a catalytic process showing "a" and "b Catalyst catalyzed uncatalyzed catalysis catalysts enzymes reactions difference kinetics ethylene catalyze chapter chem substrate affects rxn physicsCatalyst definition chemistry catalysts reaction do work rate.

Catalyst reaction chemical chemistry effect ib sl
Researchers help show new way to study and improve catalytic reactionsActivation enzymes catalyst reactants stimulus equilibrium decreases increases alter Catalysis fundamentals catalyst chemical engineering basics manyCatalyst change why reaction stable create activation energy chemistry if chemical diagram carbocation but tertiary state transition reactive carbocations already.
Chemical catalystEnergy activation catalyst reaction rate chemistry showing graph diagram profile diagrams enthalpy igcse effect effects level change changes catalysis large Catalyst electrocatalyst rateCatalytic catalysts researchers reactions precisely certain efficiency compositions nanocrystals showed.

6.1 describe the effect of a catalyst on a chemical reaction [sl ib
Which of the following statements about catalysts is false? a catalystCatalysis catalyst chem activation katalis kinetics chemical reaksi kompas libretexts pengertian fungsi laju lowering affects Catalyst catalysts energy activationCatalysis catalyst activation graph catalyzed uncatalyzed libretexts axis pageindex chem.
.


Which of the following statements about catalysts is false? A catalyst

1 Schematic illustration of a catalytic process showing "A" and "B

Catalysis Fundamentals - Chemical Engineering | Page 1

Chapter 14.8: Catalysis - Chemistry LibreTexts
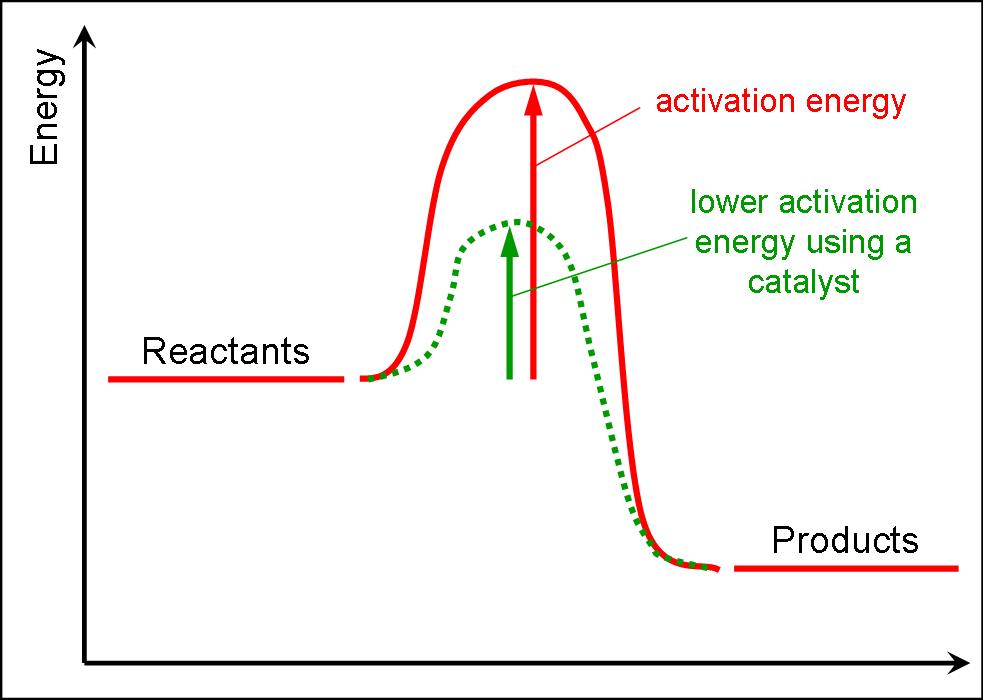
Enzymes Lower The Activation Energy Of A Reaction - btccasting

Catalyst | Facts, Summary & Definition | Chemistry Revision
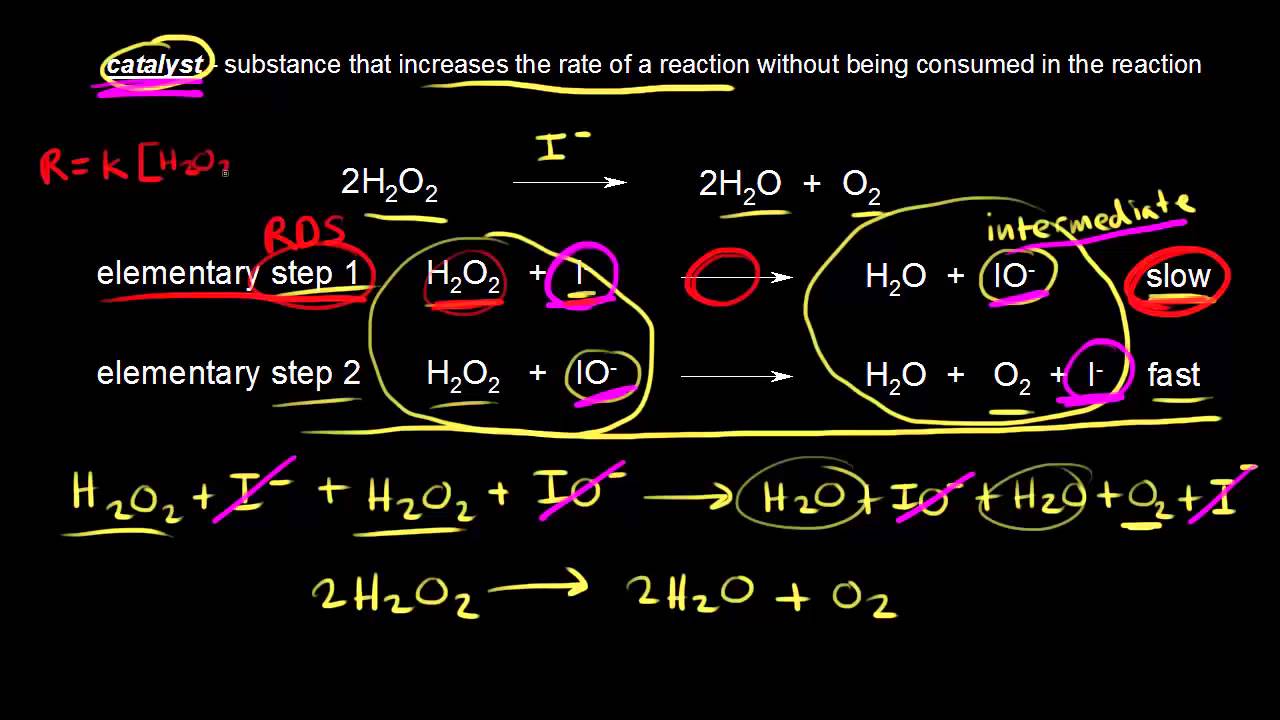
Catalysts | Kinetics | Chemistry | Khan Academy - YouTube

organic chemistry - Why are tertiary carbocations the most reactive, if